Atlas of Atomic Nuclear Structures according to the Basic Structures of Matter Supergravitation Unified theory
Stoyan Sarg
Abstract The Atlas of Atomic Nuclear Structures (ANS) is one of the major output results of the Basic Structures of Matter (BSM) theory, based on an alternative concept of the physical vacuum. The atlas of ANS contains drawings illustrating the structure of the elementary particles and the atomic nuclei. While the unveiled physical structures of the elementary particles exhibit the same interaction energies as the Quantum Mechanical models, they permit revealing the spatial configurations and size of the atomic nuclei, the atoms, and the molecules. The unveiled structural features allow an understanding cause of radioactivity, isotope stability, classical explanation of the nuclear magnetic resonance, and bond directions in atoms in molecules. The proposed physical models could find applications in different fields, such as chemistry, nuclear reaction, nanotechnology and biomolecules.
Tables of contents Page
1. Introduction 2
2. Part I: Structure of the elementary particles I-1
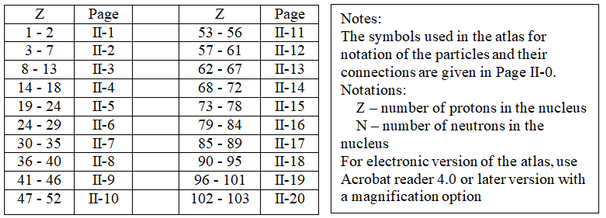
3. Part II: Atomic nuclear structures of the elements (Table 1)
4. Nuclear views for some selected elements II-21
Table 1. The elements are referenced by their Z number
References:
also in electronic archive of National Library of Canada, AMICUS No. 27105955), (2002)
2. S. Sarg © 2001, “Atlas of Atomic Nuclear Structures”, monograph. Archived in the National Library of Canada (April 2002) (AMICUS No. 27106037) Canadiana: 2002007655X, ISBN: 0973051515. Classification: LC Class no.: QC794.6; Dewey: 530.14/2 21
3. S. Sarg, New approach for building of unified theory about the Universe and some results, http://lanl.arxiv.org/abs/physics/0205052 (2002)
4. Stoyan Sarg, Atlas of Atomic Nuclear Structures (first edition) https://vixra.org/abs/1107.0031
Introduction
The Atlas of Atomic Nuclear Structures (ANS) is one of the major output results of the Basic Structures of Matter (BSM) theory, based on an alternative concept of the physical vacuum. The revealed structure of space and elementary particles permitted unveiling the physical structure of the atomic nuclei. The spatial arrangement of the protons and neutrons in the atomic nuclei allows explanation of the angular positions and freedom of the chemical bonds. The obtained features are in good agreement with the VSEPR model used in chemistry. The analysis of different properties of the elements, such as a first ionization potential, X-ray properties, Hunds rules, Pauli exclusion principles, oxidation number and many others provide a strong supporting evidence about the proposed physical models of the nuclei. Detailed arguments about this are presented in Chapter 8 of BSM. The ANS could find verry useful applications in different fields, such as inorganic and organic chemistry, nuclear reactions, nanotechnology and biomolecules.
The atlas of ANS contains two parts. Part I illustrates the geometry and the internal structure of the basic atomic particles, built of helical structures. (The helical structures have common geometrical features. Their type and classification are shown in §2.7, Chapter 2 of BSM). Part II illustrates the three dimensional nuclear structures of the elements in a range of 1 < Z < 103, where Z is the number of protons in the nucleus. Only the stable isotopes given in the Periodic table are shown. In order to simplify the complex views of the nuclei, they are shown as plane projections of symbols (symbolic views). For this purpose two types of symbols are used: symbols for hadron particles (proton, neutron and He nucleus) and symbols for the type of the nuclear bonding of the hadrons. The symbolic views contain the necessary information for presenting the real three-dimensional structures of the atomic nuclei by different sectional views. This is demonstrated in page 21 of the atlas, where nuclear sectional views of some selected elements are shown.
The rules according to which the protons and neutrons are arranged in shells in the nuclei are discussed in Chapter 8 of BSM. The trend of consecutive nuclear building by Z-number follows a shell structure that complies strictly with the row-column pattern of the Periodic table. The periodic law of Mendeleev appears to reflect not only the Z-number, but also the shell structure of the atomic nuclei. The latter becomes apparent in the BSM analysis. The protons (deuterons) shells get stable completion at column 18 (noble gases). The separate rows of the Lanthanides and the Actinides are characterised by a consecutive grow and completion of different shells. The nuclear structures of all stable elements (isotopes) possess a clearly identified polar axis of rotational symmetry. One or more He nuclear structures are always positioned along this axis. The most abundant sub-nuclear compositions are deuterons, tritium and protons. The strong SG forces hold them together, while the proximity E-fields play a role for their orientations. The identified different types of bonds are shown in the atlas by symbolic notations. For more details, see Chapter 8 of BSM. In the same Chapter, the conditions for instability of the short-lived isotopes are also discussed. They are partly apparent from the Atlas drawings – especially for the alpha decay. The growing limit for stable high Z-number elements is apparent from the shelf completion and the obtained nuclear shape.
The electronic orbits are not shown in the nuclear drawings, but their positions are defined by the spatial positions of the protons (or deuterons). The Hund’s rules and the Pauli exclusion principle are both identifiable features related to the available positions and mutual orientations of the quantum orbits. The quantum velocity of the orbiting and oscillating electron, defines the length trace of any quantum orbit (see §3.12, Chapter 3; §7.4, Chapter 7 of BSM)